
Once ignited, a chain reaction must take place whereby fires can sustain their own heat by the further release of heat energy in the process of combustion and may propagate, provided there is a continuous supply of an oxidizer and fuel. Some fuel-oxygen mixes may require a catalyst, a substance that is not consumed, when added, in any chemical reaction during combustion, but which enables the reactants to combust more readily. For example, a flammable liquid will start burning only if the fuel and oxygen are in the right proportions. Fire cannot exist without all of these elements in place and in the right proportions. This is commonly called the fire tetrahedron. This loss of nitrogen caused by a fire produces a long-term reduction in the fertility of the soil, but this fecundity can potentially be recovered as molecular nitrogen in the atmosphere is " fixed" and converted to ammonia by natural phenomena such as lightning and by leguminous plants that are "nitrogen-fixing" such as clover, peas, and green beans.įire has been used by humans in rituals, in agriculture for clearing land, for cooking, generating heat and light, for signaling, propulsion purposes, smelting, forging, incineration of waste, cremation, and as a weapon or mode of destruction.įires start when a flammable or a combustible material, in combination with a sufficient quantity of an oxidizer such as oxygen gas or another oxygen-rich compound (though non-oxygen oxidizers exist), is exposed to a source of heat or ambient temperature above the flash point for the fuel/oxidizer mix, and is able to sustain a rate of rapid oxidation that produces a chain reaction. Also, when vegetation is burned, the nitrogen it contains is released into the atmosphere, unlike elements such as potassium and phosphorus which remain in the ash and are quickly recycled into the soil. If fire removes protective vegetation, heavy rainfall may lead to an increase in soil erosion by water. Its negative effects include hazard to life and property, atmospheric pollution, and water contamination. The positive effects of fire include stimulating growth and maintaining various ecological systems. Fire is an important process that affects ecological systems around the globe. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.įire in its most common form can result in conflagration, which has the potential to cause physical damage through burning.
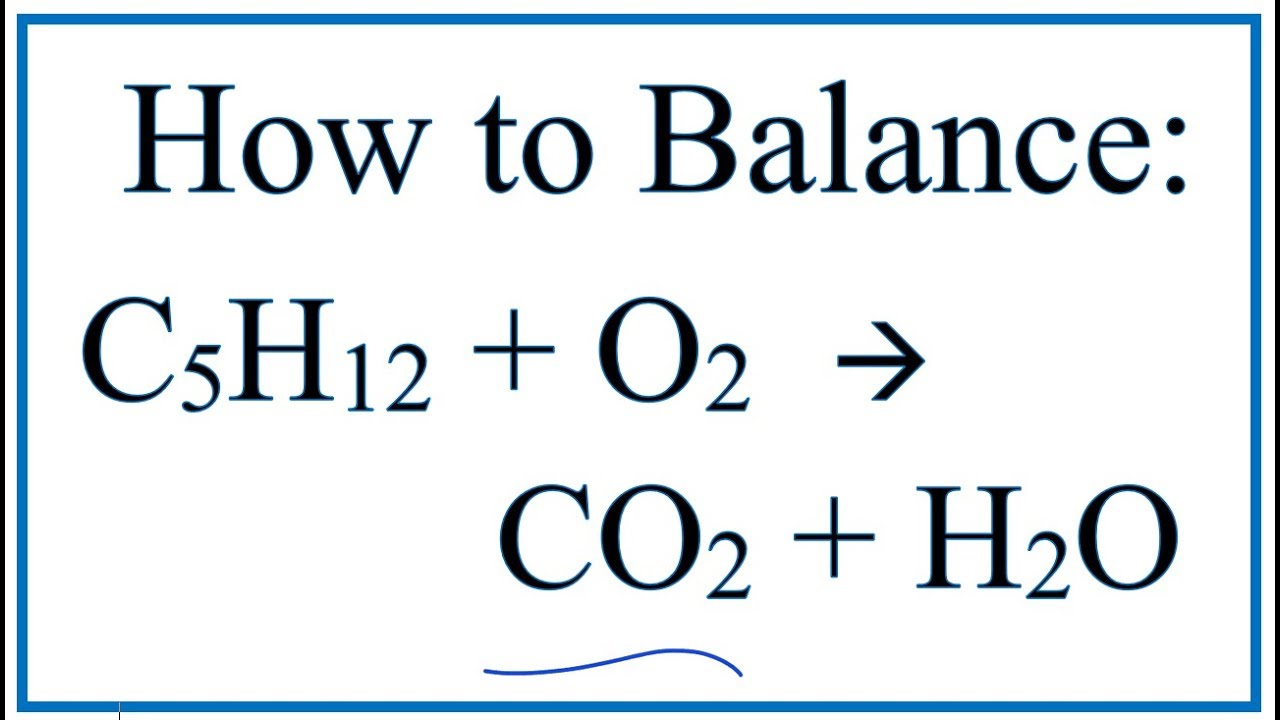
If hot enough, the gases may become ionized to produce plasma. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. The flame is the visible portion of the fire. At a certain point in the combustion reaction, called the ignition point, flames are produced. įire is hot because the conversion of the weak double bond in molecular oxygen, O 2, to the stronger bonds in the combustion products carbon dioxide and water releases energy (418 kJ per 32 g of O 2) the bond energies of the fuel play only a minor role here. Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.


 0 kommentar(er)
0 kommentar(er)
